Stem cells and Niche cells
SCGF is basically active on stem/progenitor cells. Recombinant human (rh) SCGF exhibits erythroid burst-promoting activity (BPA) and granulocyte/macrophage colony-promoting activity (GPA) on erythroid and GM progenitor cells in a primary semisolid clonal culture with erythropoietin and GM-CSF, respectively (1, 2, 6). In fact, scgf cDNA has been cloned as positive for BPA (6).
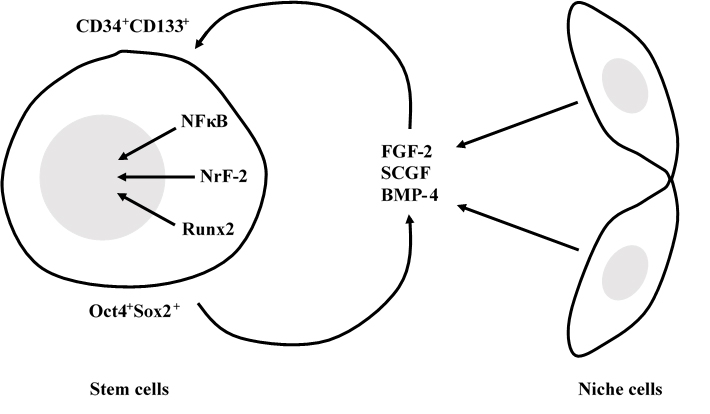
SCGF is an autocrine or paracrine mediator between stem cells and stem cell niche cells.
rhSCGF is further applied to amplify hematopoietic stem/progenitor cells (
117-
120,
Pat6-
Pat9), endothelial progenitor cells (EPC) (
117,
121-
132,
Pat10-
Pat14,227) and their common progenitor, hemangioblasts (
133,
134).
Human hematopoietic stem/progenitor cells are expanded
ex vivo in the culture of CD34
+ cells with a cocktail of 9 cytokines including rhSCGF; 30.4 X CD34+ cells, 63.9 X CD34
+CD38
- cells, 10.7 X CFCs and 2.8 X LTC-ICs (
118,
Pat7). Similar results are obtained from the culture of human PB CD133
+ cells with SCGF/VEGF with or without
flt3 L; 60 X CFU-E, 48 X CFU-GEMM, 59 X CFU-GM, 99 X CFU-G, 1356 X CFU-M, 1843 X CFU-EC, and SRCs are demonstrated in the BM cells of irradiated NOD/SCID mice (
117,
119,
Pat6,
Pat8,
Pat9).
Oct4+ cells are maintained through the culture of umbilical cord blood (UB)-derived stem cell marker-sorted cells with SCGF/
flt3 L(
120). Human umbilical cord blood-derived CD133
+ cells contain equal CFU-GM and one fourth BFU-E detected in the semisolid culture with SCGF+G-CSF and SCGF+Epo, respectively, as compared with CD34
+ cells (
288).
When human UB-derived CD34
+ cells are cultured with 10 ng/ml
c-kit L and 100 ng/ml SCGF for 7 days, CD34
+ cells decline to 60% of the starting level (
420).
EPCs are originally reported to be amplified in the culture of PB CD133
+ cells on the fibronectin-coated plates containing SCGF/VEGF (
117). This method is modified to be applicable to the culture of CD34
+ and/or CD133
+ cells from PB, BM and UB with
flt3 L (
123,
128,
130,
Pat10),
c-kit L/FGF-2 (
122,
124,
Pat13) or FGF-2/IGF-1/EGF (
131) in addition to the basic combination of SCGF/VEGF. Endothelial cells (EC) recruited from EPCs amplification can be used to (1) treat damaged vessels of ischemic, autoimmune (
122) and septic (
125) vascular diseases, (2) coat coronary stents (
123,
Pat11), artificial vessels (
126,
Pat11) and cardiac valves (
Pat11) to prevent restenosis and thrombosis, (3) be transfected with VIII factor gene for hemophiliac patients (
124,
Pat13), (4) carry toxins to the tumor vasculature (
122), (5) be radiolabeled to detect metastasis (
Pat11), and (6) endothelialize poly 4-methyl-1-pentene gas exchange membrane (
304).
Hemangioblasts are common progenitor cells capable of differentiating into the hematopoietic and endothelial cell lineages, and have been thought to appear only in fetal life. However, human adult CD133
+ cells contain hemangioblasts after
ex vivo expansion in the presence of SCGF/VEGF/
flt3 L (
133,
134).
Human SCGF is species cross-active on mouse hematopoietic progenitor cells (
135) and rat EPCs (
131,
136).
There have been some reports about
in vitro activity of SCGF on other stem/progenitor cells.
(1) Human SCGF is not chemotactic for MSCs (
137,
Pat15), but used to prepare them (
Pat16).
(2)
Scgf gene expression is highly down-regulated in the RelA
-/- (a component of NF-κB) hES cell-derived MSCs, and they exhibit defective self-renewal ability (
592). Knockdown of wild type hMSCs with
scgf shRNA abrogates their proliferative potential, indicating that
scgf is a mandatory driver for MSC proliferation.
(3) Partial hepatectomy mobilizes CD133
+CD45
+CD14
+ cells in the peripheral blood of healthy liver donors, which have a GM colony-forming ability and differentiate to hepatic cells in the presence of SCGF/HGF/EGF/FGF-4 (
138).
(4) Combination of PDGF-BB/EGF/SCGF/TNF-α/IL-1β is optimal for proliferation of multipotential adipose stem cells (ASCs;
Pat17,
Pat18). The basal AR8 medium has been developed for low human serum (0.5%) or serum-free culture of human ASCs, and contains several hormones and cytokines in addition to 1ng/ml SCGF in DMEM/F12 medium (
267). Human ASCs grown in the AR8 medium with 1% human serum can be applied to diabetic ulcers in leptin receptor-deficient (
db/db) mice and accelerate wound healing (
268).
(5) A population of CD133
+CD34
+CD45
+CD14
+ cells in the human corneal stroma is induced to macrophages and fibroblasts, but not to endothelial cells in the presence of SCGF/VEGF (
139).
(6) SCGF is identified one of anchorage-independent tumor growth factors in the bovine serum (
140). See also "
Disease" section for tumor growth activity of SCGF.
SCGF interacts with splice variant 2 but not with splice variant 1 of methionine adenosyltransferase 2β up-regulated in colon cancer cell line, RKO (
257), which could regulate transcription and give a growth advantage to cancer cells.
Mouse SCGF restores hematopoietic defect in CDH5-MAPK mice and irradiation-induced myelosuppression in wild type mice.
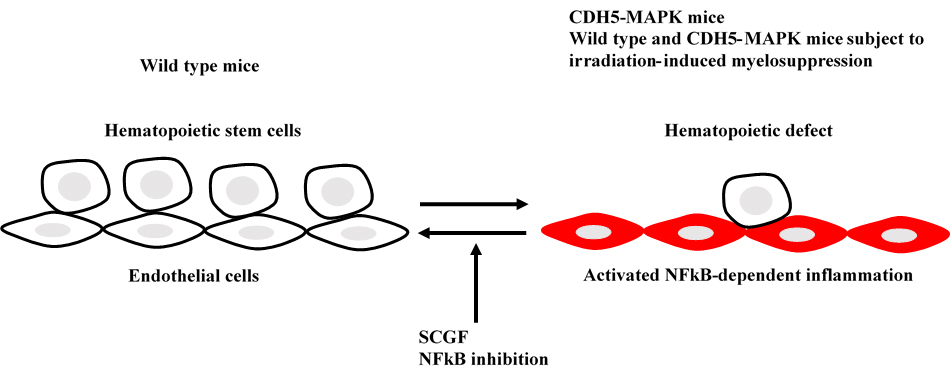
Selective MAPK-activation in bone marrow endothelial cells from CDH5-MAPK mice up-regulates MAPK-downstream NFkB to induce perivascular niche inflammation (
616,Pat34). Disruption of endothelial cells leads to profound hematopoietic defect including exhaustion of hematopoietic stem (HSCs)/progenitor cells, decreased quiescent HSCs, elevated HSC apoptosis, decreased competitive repopulating ability of HSCs and decreased peripheral blood cells with myeloid-biased output. Plasma SCGF level is dramatically down-regulated in CDH5-MAPK mice, which is normalized by NFkB inhibition. Mouse SCGF resolves hematopoietic and vascular defects when administered to CDH5-MAPK mice or irradiated wild type mice.
Mouse BM scgf
+LepR
+ periarteriolar stromal cells are actively proliferating short-lived osteogenic progenitors (
643). In response to exercise or mechanical loading, they exhibit SCF-mediated maintaining activity for adjacent IL-7Rα
+lin
- common lymphoid progenitors without affecting mature B and T cells and myeloid stem/progenitor cells.
Primitive hematopoietic stem/progenitor cells differentiated
in vitro from H1 human ES cells express
scgf gene (
676).
SCGF promotes human B cell survival alone and synergistically with BAFF (
678). Coculture of human CD20
+ B cells with autologous CD14
+ monocytes gives rise to 4-fold live CD19
+ B cells compared to the culture of B cells without monocytes. Antibodies against SCGF and/or BAFF secreted in the coculture supernatants block B cell survival up to 50% level of the coculture. B cell survival is promoted in the culture of B cells with rhSCGF and/or rhBAFF. Monocytes promote B cell survival mainly via BAFF and SCGF.
Osteogenesis
Mouse
scgf induces both fetal and adult osteogenesis.
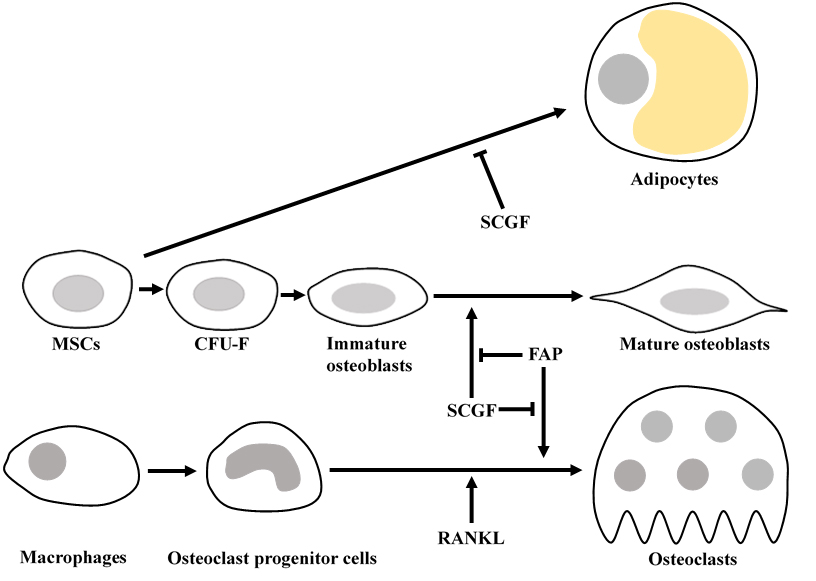 Scgf
Scgf acts on Sp7 (Osterix)
+, Runx2
+, or Col1a1
+ immature osteoblasts to differentiate into Ibsp
+ or Dmp1
+ mature osteoblasts (
537,
Pat30).
Scgf-/- mice appear to be born normally, but develop femoral and vertebral osteoporosis with aging. MicroCT analysis detects osteogenic impairment, e.g. decreased trabecular bone mass, number, thickness, density and increased spacing, however bone resorption is not affected in
scgf-/- mice. Bone marrow cells from
scgf-/- mice contain normal numbers of LepR
+CD45
-Ter119
-Tie2
- MSCs, CD51
+ MSCs and CFU-F. Osteogenic culture of
scgf-/- MSCs generates normal counts of Sp7 (Osterix)
+, Runx2
+, or Col1a1
+immature osteoblasts but decreased counts of Ibsp
+ or Dmp1
+ mature osteoblasts. Chondrogenic and adipogenic differentiation is normal in the culture of
scgf-/- MSCs. The impaired osteogenic differentiation of
scgf-/- MSCs is rescued by addition of rmSCGF to the culture. Subcutaneous administration of rmSCGF to
scgf-/- mice with or without experimental bone fracture promotes improvement of osteoporosis. Osteoporosis in ovariectomized or dexamethasone-treated wild type mice is successfully prevented or ameliorated by
in vivo administration of rmSCGF. rhSCGF similarly promotes osteogenic differentiation in the culture of human MSCs.
Scgf has an osteogenic function to maintain adult skeleton. SCGF partly mediates an osteogenic effect of PTH, for PTH-promoted bone formation is attenuated in scgf
-/- mice administered with human Teriparatide (N-terminal 34 aa polypeptide of PTH) compared to similarly treated wild type mice (
652). Four-week-old but not younger or older scgf
-/- mice exhibit 3-4% shorter femur length and a fewer chondrocytes in the growth plate than those in wild type control mice (
690). rmSCGF activates Wnt pathway in the growth plate chondrocytes to promote their osteogenic differentiation
in vitro, and facilitates chondrocyte proliferation in the growth plate and bone elongation
in vivo. When resting chondrocytes in the growth plate of PTHrP-creER; Ptch1
fl/fl conditional KO mice are specifically activated for Hedgehog signaling, their
PTHrP and
scgf-upregulated skeletal stem cell-like subsets differentiate and migrate to proliferative chondrocytes then to hypertrophic chondrocytes, and eventually to osteoblasts (
698).
Senescent osteogenesis is suppressed by interaction of fibroblast activation protein (FAP) with SCGF. Immunoprecipitation and mass spectrometry analysis of scgf-FLAG-expressed mouse MC3T3-E1 cells identifies 85kDa FAP as SCGF-binding protein (
630). FAP is a proline-specific serine protease and expressed in MSCs and osteoblasts. FAP protease activity is suppressed in scgf- and FAP-overexpressed mouse HEK293T cells, and FAP is increased in scgf
-/- mice. Senescent osteogenesis is promoted with increased osteoblasts and decreased osteoclasts in FAP
-/- mice while young FAP
-/- mice grow normally. FAP-selective inhibitor N-acyl-Gly-Pro-boronic acid promotes osteogenesis in untreated wild type mice and mitigates osteoporosis in ovariectomized wild type mice. Enhanced bone formation and suppressed bone resorption by FAP inhibitor is associated with up-regulation of Wnt-related genes and down-regulation of NF-κB-related genes in MSCs, respectively. Bone calcification in fetal zebrafish is repressed by inhibiting scgf while facilitated by inhibiting FAP. Osteogenesis and osteoclastogenesis are regulated by interaction between scgf and FAP.
SCGF has been thought to bind integrins (ITGs) through RGD motif sequence. Of human ITG α4β1, α9β1, α10β1, α11β1, αVβ1, αVβ3, αIIbβ3 and αMβ2 tested, human SCGF selectively binds ITGα10β1 and ITGα11β1 with similar high affinity as human pro-collagen 1α through RGD (
594). Mouse SCGF acts on human and mouse MSCs to induce osteogenic differentiation despite lacking RGD through activated Wnt signal transduction, i.e. phosphorylation of GSK3, accumulation of nuclear β-catenin and up-regulation of Wnt target genes of Alp, Axin2, Lef1 and Runx2. ITGα10β1 and ITGα11β1 are not RGD-recognizing integrins and mouse SCGF lacks RGD, indicating that SCGF binds integrins through other mechanisms than RGD.
SCGF is highly enriched in human umbilical cord (UC)-MSCs-derived exosomes compared to parental MSCs. Human SCGF-rich UC-MSCs-derived exosomes increase trabecular bone mass, and decrease BM fat accumulation and osteoclasts when administered intravenously to mice with ovariectomy- or disuse (hindlimb-unloading by tail suspension)-induced osteoporosis (615). Human scgf+UC-MSCs-derived exosomes enhance and inhibit in vitro differentiation of mouse BM-MSCs into osteoblasts and adipocytes/osteoclasts, respectively, but human scgf-(silenced with scgfshRNA) UC-MSCs-derived exosomes exhibit no such bone effects. Tripeptide PSP up-regulates scgf in vascular endothelial cells to promote osteogenesis of mouse BM-MSCs (708). Differentiation of mouse Raw264.7 osteoclast progenitor cellsinto osteoclasts in the presence of RANKL is blocked by human scgf+but not scgf-UC-MSCs-derived exosomes.
Methylprednisolone-induced osteonecrosis of the rat femoral head is alleviated by oral administration of simvastatin and/or intramedullary
injection of rhSCGF (714). Adipogenesis and osteogenesis of human MSCs is inhibited and promotedin vitro, respectively, by simvastatin-upregulated SCGF. These findings indicate that statins and/or SCGF are applicable to therapeutic use for hyperlipidemia-associated
osteonecrosis.
SCGF facilitates dose-dependentlyin vitro osteogenesis of porcine BM-MSCs (622).
Androgenetic haploid mouse ES cells with
scgf-targeted mutagenesis are injected into mature oocytes to yield
scgf-/- or chimeric
scgf+/- mice (
604). The
scgf-mutated newborn mice exhibit bone abnormalities, e.g. size reduction, impaired calcification and hypoplastic skull. Mouse
scgf exerts osteogenic activity during fetal development.
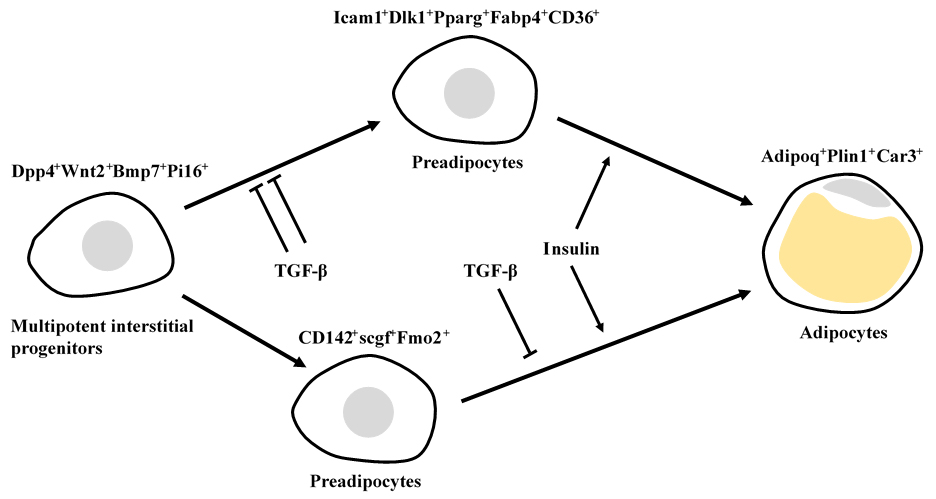
Adipogenesis
An adipogenic differentiation consists of 4 distinct cell types in mouse white adipose tissue: Dpp4
+Wnt2
+Bmp7
+Pi16
+ multipotent interstitial progenitors in the reticular interstitium, Icam1
+Dlk1
+Pparg
+Fabp4
+CD36
+ and CD142
+scgf
+Fmo2
+ preadipocytes in the perivascular niche, and Adipoq
+Plin1
+Car3
+ adipocytes (
602). Icam1
+ and CD142
+ preadipocytes but not Dpp4
+ progenitors differentiate to adipocytes in response to insulin. TGF-β inhibits an adipogenic differentiation of Dpp4
+ progenitors but not Icam1
+ preadipocytes, and exerts a moderate inhibitory effect on CD142
+ preadipocytes. In contrast to mouse adipose tissue, there is no CD142
+scgf
+Fmo2
+ preadipocyte subpopulation in human adipose tissue. Human Dpp4
+progenitors and Icam1
+ preadipocytes also express CD142, scgf and Fmo2. CD142
+scgf
+ adipogenesis regulators (Aregs) are localized to perivascular niche in subcutaneous white adipose tissue (
657), and inhibit differentiation of ICAM1
+VAP1
+ preadipocytes to adipocytes. Retinoic acid (0.01-1μM) up-regulates
scgf gene expression in mouse CD142
- adipose stem/progenitor cells, but their adipogenesis is not inhibited by exogenous rmSCGF but by F3 (CD142), MGP or GDF10, all of which are signature proteins expressed in CD142
+ Aregs (
681).
R-spondin 2 secreted by mouse CD142
+scgf
+Fmo2
+Gdf10
+Igfbp3
+ Areg cells binds to Lgr4 of CD142
-Cd55
+Dpp4
+ early adipocyte progenitor cells and inhibits their adipogenic differentiation (
665).
Mouse Pdgfra
+Bace2
+Vipr2
+scgf
+ stromal cells reside close to adventitia of thoracic aorta in the perivascular adipose tissue (
648).
Mouse scgf
+Cpe
+CD142
- subpopulation of perivascular adipose-derived MSCs could potentially differentiate to smooth muscle cells (
662).
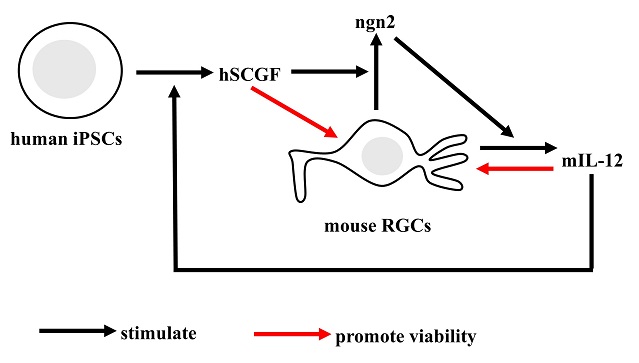
 Nerve cells
Nerve cells
SCGF is secreted by human iPSCs in response to mouse retinal ganglion cells (RGCs)-derived IL-12 and promotes RGC viability through ngn2 up-regulation (
710).
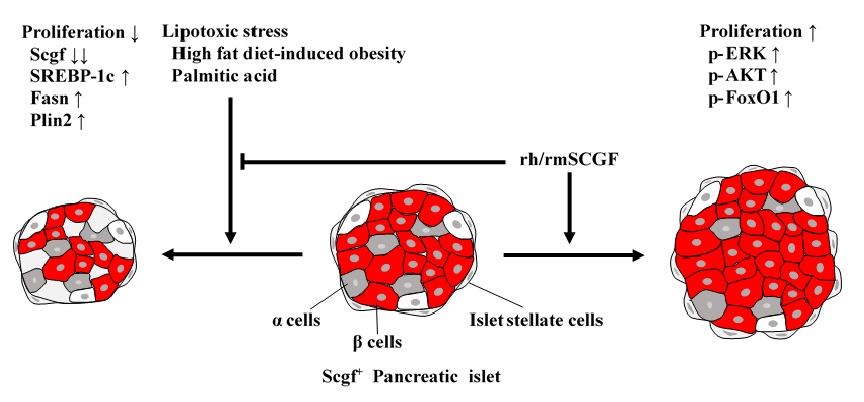
 Pancreatic islet cells
Pancreatic islet cells
Lipotoxic stress, e.g. high fat diet-induced obesity and
in vitro treatment with palmitic acid, down- and up-regulates
scgf and lipid metabolism-related SREBP-1c, Fasn, Plin2 genes in mouse pancreatic islet stellate and β cells, respectively to suppress their proliferation (
609). rh/rmSCGF mitigates the lipotoxicity on mouse islet cells, and further activates ERK, AKT, FoxO1 to stimulate their proliferation. Human islet α and β cells but not human β cell line EndoC-βH1 cells express
scgf gene. Exogenous rhSCGF up-regulates
MAFA and
PDX1 in vitro to promote proliferation of human β cells and EndoC-βH1 cells with facilitated insulin secretion (
691). Exogenous rhSCGF partially improves β cell function of EndoC-βH1 cells from palmitate-induced lipotoxicity.
 Germline stem cells (GSCs)
Germline stem cells (GSCs)
SCGF, or daidzein via
scgf up-regulation, activates Akt signaling pathway to promote viability and proliferation of mouse female germline stem cells (FGSCs)
in vitro (
680).
Scgf knockdown suppresses Akt phosphorylation and proliferation of FGSCs, and the inhibition is not rescued by daidzein.
Cell adhesion to endothelial cells, Microenvironmental vasculogenesis and angiogenesis
SCGF alone has no angiogenic ability
in vitro, but facilitates endothelial proliferation in the presence of VEGF or FGF. SCGF over-produced by H1299 or HCC827 lung cancer cells exhibits chemotactic activity on ambient vascular endothelial cells and promotes tumor growth with enhanced angiogenesis when cancer cells are transplanted into nude mice (
671). The chemotactic activity is mediated through integrin activation different from conventional FAK or p130 signaling. SCGF-promoted tumor growth is suppressed with
scgf shRNA treatment. SCGF suppression could be applied to a novel anti-cancer therapy.
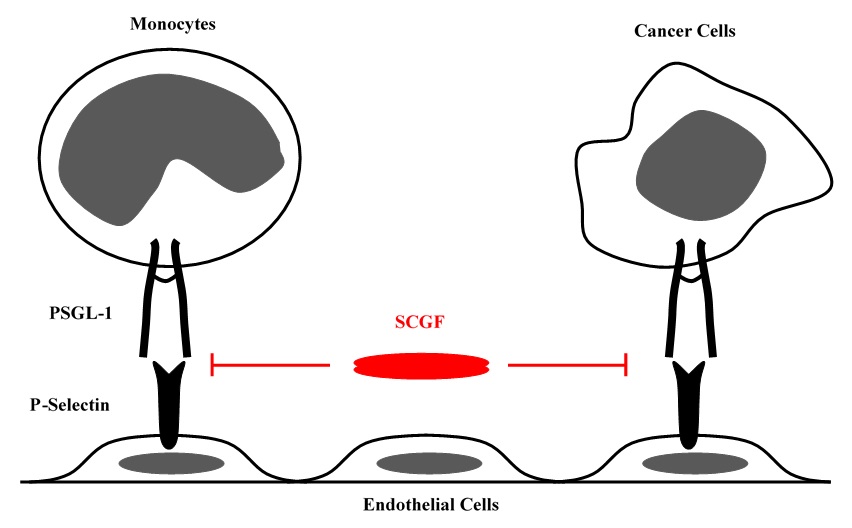
SCGF is an antagonist of P-selectin glycoprotein ligand-1 (PSGL-1/CD162), i.e. inhibits PSGL-1/CD162-selectin-mediated U937 cell adhesion to EA.hy926 endothelial cells and breast cancer MCF7 or MDA-MB231 cell adhesion to culture plate (
Pat32).
Human primitive CD34
+CD38
- hematopoietic progenitor cells coexpress PSGL-1/CD162. They are induced to be apoptotic and CFU-GMs are completely inhibited to proliferate by immobilized or soluble P-selectin in the culture stimulated with IL-3, IL-6, G-CSF and SCF (
574). It can partially explain SCGF's GPA or BPA that SCGF blocks such PSGL-1/CD162-selectin-mediated inhibition of hematopoietic progenitor cells.
 In vivo
In vivo effect of SCGF is studied using 8-10 week old C57BL/6J mice as compared with saline control (
Pat19);
(1) Daily administration of rhSCGF 5μg for 7 days decreases CD34
+ or
flk1+ cells in the peripheral blood, and suppresses EC growth on the subcutaneously transplanted Matrigel.
(2) Daily administration of rhSCGF 5μg for 21 days induces neutropenia and monocytopenia but not anemia and thrombocytopenia, and suppresses growth of B16 melanoma subcutaneously transplanted.
(3) When rhSCGF 5μg is given daily for 1, 4 and 7 days, EPC growth on the vitronectin-coated slide glasses is dose-dependently suppressed.
(4) EPC-rich human microvascular endothelial cells (HMVEC) but not mature human umbilical vein endothelial cells (HUVEC) are stimulated to proliferate in response to rhSCGF. SCGF has little effects on vascular tube-formation of HMVECs and HUVECs.
(5) Human PB-MNCs are not stimulated to grow into endothelial cell lineage on the fibronectin-coated plates containing rhSCGF.
(6) When rhSCGF is added to the lower chamber, human BM-MNCs and HMVECs but not HUVECs and mature ECs from EPCs migrate from the upper chamber through limiting membrane to the lower chamber.
(7) Tripeptide PSP promotes proliferation and angiogenesis of HUVECs and MAECs to up-regulate their
scgf gene expression (
708).
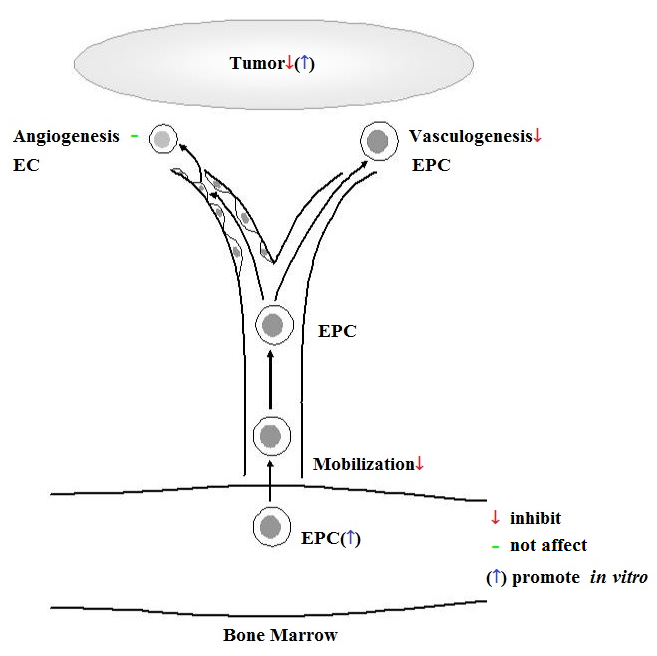
SCGF inhibits EPC mobilization from bone marrow, vasculogenesis and tumor growth in vivo, while accelerating proliferation of tumor cells in vitro.
Taken together, SCGF inhibits vascular formation and thereby tumor growth. SCGF specifically inhibits vasculogenesis, i.e. mobilization of stem/progenitor cells or EPCs to the peripheral blood, but not angiogenesis by pre-existing mature ECs. SCGF could proliferate and lock stem/progenitor cells within the BM microenvironment.