A molecular mass (Mr) of purified rhSCGF-α is 45 kDa on SDS-PAGE (below) (
11) (Table and Figure), relatively larger than the calculated Mr of 33,534 Da. Digestion of rhSCGF with endo-O-glycosidase and sialidase reduces the Mr of 45 kDa to 40kDa (Figure; lane 5), implicating that rhSCGF from CHO cells is modified by a possible post-translational O-glycosylation including sialic acids, while no N-glycosylation site is deduced from
scgf cDNA. SCGF is actually demonstrated to be O-glycosylated at Thr69 by N-acetylgalactosamine (
433).
|
Molecular mass (kDa) |
CHO cell product |
E. coli product |
hSCGF-α |
|
|
hSCGF-β |
|
|
Isoelectric point of SCGF is predicted in silico at Phosphosite (Table).
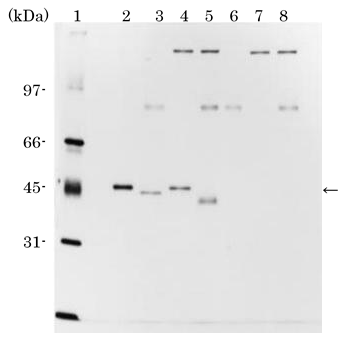
Silver-stained SDS-PAGE of purified rhSCGF-α after
digestion with endo-O-glycosidase and/or sialidase. (
11)
Lane 1, molecular mass markers; lane 2, purified rhSCGF-α; lane 3, rhSCGF-α digested with sialidase; lane 4, rhSCGF-α digested with endo-O-glycosidase; lane 5, rhSCGF-α digested with endo-O-glycosidase in the presence of sialidase; lane 6, sialidase alone; lane 7, endo-O-glycosidase alone; lane 8, endo-O-glycosidase and
sialidase. An arrowhead indicates the rhSCGF-α band.
Protein
Recombinant SCGF is produced from pAGE-SCGF-α-transfected CHO cells (
11) or
E. coli, the latter of which is commercially available (see
Materialssection).
The NH2-terminal aa sequence of rhSCGF-α is ARGAEREWEG, alanine of which corresponds to the 22nd A of the aa sequence deduced from scgf cDNA. Therefore a 21 aa signal peptide MQAAWLLGALVVPQLLGFGHG is removed to make up secreted mature SCGF (11).
SCGF interacts with splice variant 2 but not with splice variant 1 of methionine adenosyltransferase 2Β up-regulated in colon cancer cell line, RKO (
257), which could regulate transcription and give a growth advantage to cancer cells.
Listed below are accession numbers of SCGF in the protein database.
SCGF is actually stable in a trimeric form (data not shown) as is tetranectin molecularly akin to SCGF (
12).
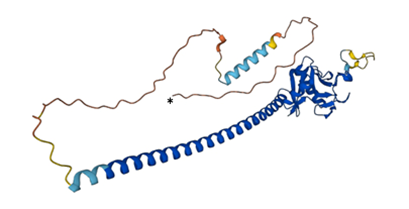
Homotrimer forms a triple α-helical coiled coil with a consensus CRD structure of 6 β-strands, 2 α-helices and 4 Ca
2+-harboring loops (
14-
16).
3D structures of human, mouse and rat SCGF have been deposited in AlphaFold Protein Structure Database.
← Human SCGF (*N-terminal) (modified from
AlphaFold)